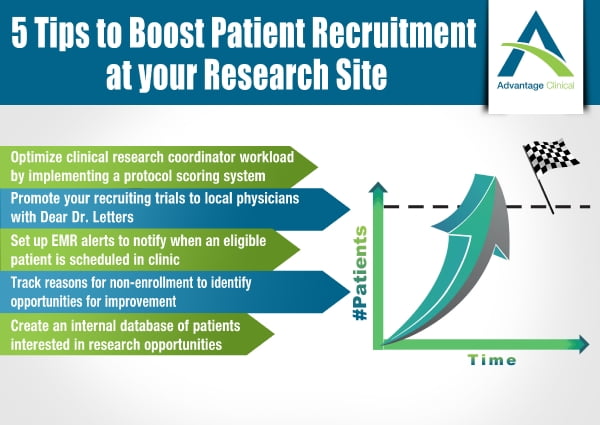
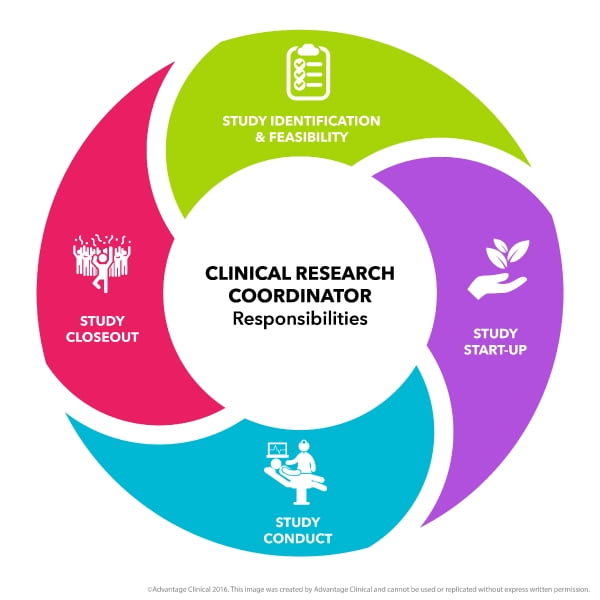
Home / Blog
Blog
- May 30, 2024
- May 3, 2024
- May 2, 2024
- May 1, 2024
- September 10, 2018
- June 6, 2018
- April 9, 2018
- March 22, 2018
- March 22, 2018
- January 18, 2018
- April 2, 2017
- March 28, 2017
- January 15, 2017
- January 10, 2017
- January 4, 2017
- November 15, 2016
- November 6, 2016
- November 1, 2016
- October 1, 2016
Contact us for a consultation today