Good Clinical Practice GCP E6 R2 Update The largest addition to Good Clinical Practice in Release 2 is the addition of Quality Management ,section 5.0, which falls under Sponsor guidance. Although this section of the guidance is directed to the sponsor audience,...
Investigator Oversight: Changes in Good Clinical Practice November 2016 Update
In the first update to Good Clinical Practice E6 since 1996, Release 2 introduced 26 major additions to the gold standard for the conduct of clinical research. Release 2 was finalized in November 2016, sites and sponsors must now must bring themselves into compliance...
GCP E6 R2 Addendum; Impacts for Investigators and Clinical Research Sites
Adopted in November 2016, the second revision to ICH Good Clinical Practices E6 R2, is the biggest revision to GCP in 20 years. The addendum was crafted partly in response to the concerns raised from hundreds of international GCP regulatory inspections. Some of the...
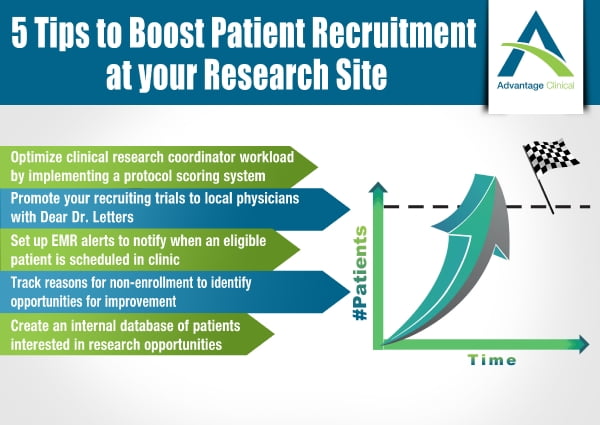
5 Tips to Boost Patient Recruitment at your Research Site
As a clinical research site you are undoubtedly constantly being approached by your sponsors inquiring about enrollment at your centre. Being a site which can efficiently and consistently meet recruitment quotas is a stellar quality and will have sponsors knocking on...
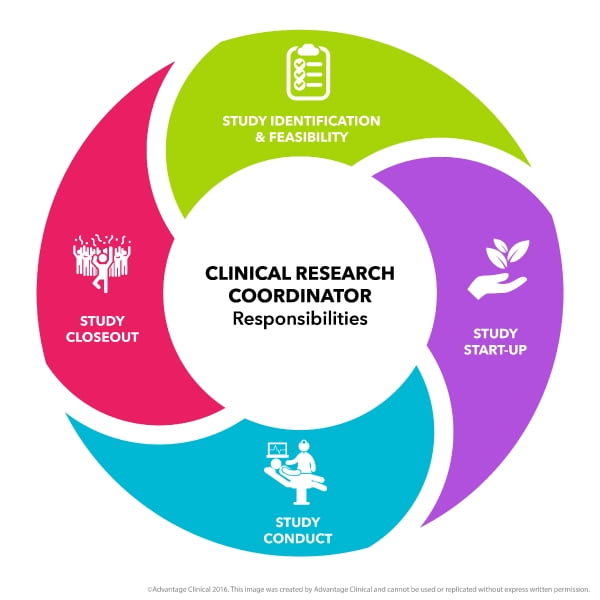
Protocol Scoring and Determining Study Load per Clinical Research Coordinator
Due to the increasing complexity of clinical research protocols and the need to justify staffing, it is critical for clinical research sites to have methods to evaluate, quantify,