CRO vendor selection pathway. Utilizing vendor scorecards to choose the right delivery partner.
Understanding the CRO Selection & Management Process
Small emerging biopharma companies typically choose a contract research organization (CRO) based on several key factors, including expertise, capabilities, reputation, cost, and flexibility. Below is an overview of how these companies might go about selecting a CRO and what the sales cycle might look like:
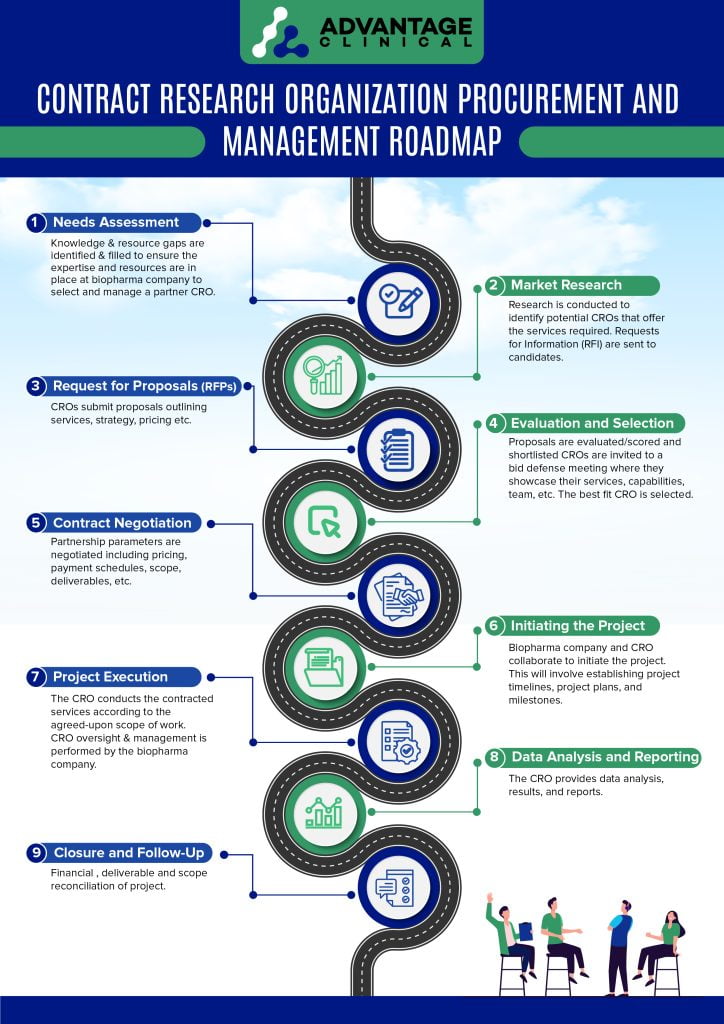
1 – Needs Assessment
The biopharma / biotechnology company assesses its specific needs for research and development support,
such as preclinical studies, clinical trials, regulatory consulting, or manufacturing services.
Knowledge gaps are filled at the biopharma company through consultants, staff & vendors to ensure the expertise and resources are in place to select and manage a partner CRO.
2 – Market Research:
Research is conducted to identify potential CROs that offer the services you require. The procurement / selection team may send a general Request for Information (RFI) to these CROs.
RFIs are reviewed to ensure that the prospect CROs are a good fit for the project.
3 – Request for Proposals (RFPs):
RFPs are now issued to multiple CROs, outlining your requirements, timelines, and potentially budget constraints.
The RFP process allows the your company to compare offerings from different CROs and negotiate terms.
4 – Evaluation and Selection:
Proposals are evaluated from CROs based on factors such as expertise, track record, quality of services, cost, and compatibility with its project goals. This is best organized via a vendor scorecard tailored to your company’s needs, goals and objectives.
Shortlisted CROs are invited to a Bid Defense meeting where they showcase their services, capabilities and team.
5 – Contract Negotiation:
Once a CRO is selected, the biopharma company enters into contract negotiations to finalize terms and conditions.
Negotiations may cover pricing, payment schedules, project timelines, deliverables, intellectual property rights, and other relevant aspects of the partnership.
6 – Initiating the Project:
Once the contract is signed, the biopharma company and the CRO collaborate to initiate the project.
This may involve establishing project timelines, project plans, and milestones.
7 – Project Execution:
The CRO conducts the contracted services according to the agreed-upon scope of work.
Throughout the project, the biopharma company will have regular communication with the CRO to monitor progress and address any issues that arise. CRO management is key to long term mutually beneficial success.
8 – Data Analysis and Reporting:
After completing the project activities, the CRO provides the biopharma company with data analysis, results, reports and any other deliverables as outlined in the contract.
The biopharma company reviews the findings to inform decision-making and regulatory submissions.
9 – Closure and Follow-Up:
Once the project is completed, the biopharma company and the CRO close out the engagement and get ready to move to the next phase of research.
The companies may conduct a post-project review to assess performance and identify areas for improvement.
Summary
The sales cycle for CROs can vary in duration depending on factors such as the complexity of the project, the decision-making process of the biopharma company, and the negotiation timeline. It can range from several weeks to several months, especially for larger and more complex projects. Building strong relationships, demonstrating expertise, and providing competitive pricing are crucial for CROs to succeed in winning business from small
emerging biopharma companies.
Working with Advantage Clinical can help you navigate this process and ensure you find the right partner for your project. We have developed robust processes to evaluate CRO candidates for fit with your organization and oversee their performance throughout the life of the projects.