CRO Management and Oversight during clinical trials
Managing the CRO Through the Clinical Development Journey
Monitoring a Contract Research Organization’s (CRO) performance throughout the life of a clinical trial is crucial to ensure that the trial is conducted efficiently, ethically, and in compliance with regulatory requirements. Here are some ways in which a sponsor or sponsor partner (Advantage Clinical) should monitor a CRO’s performance:
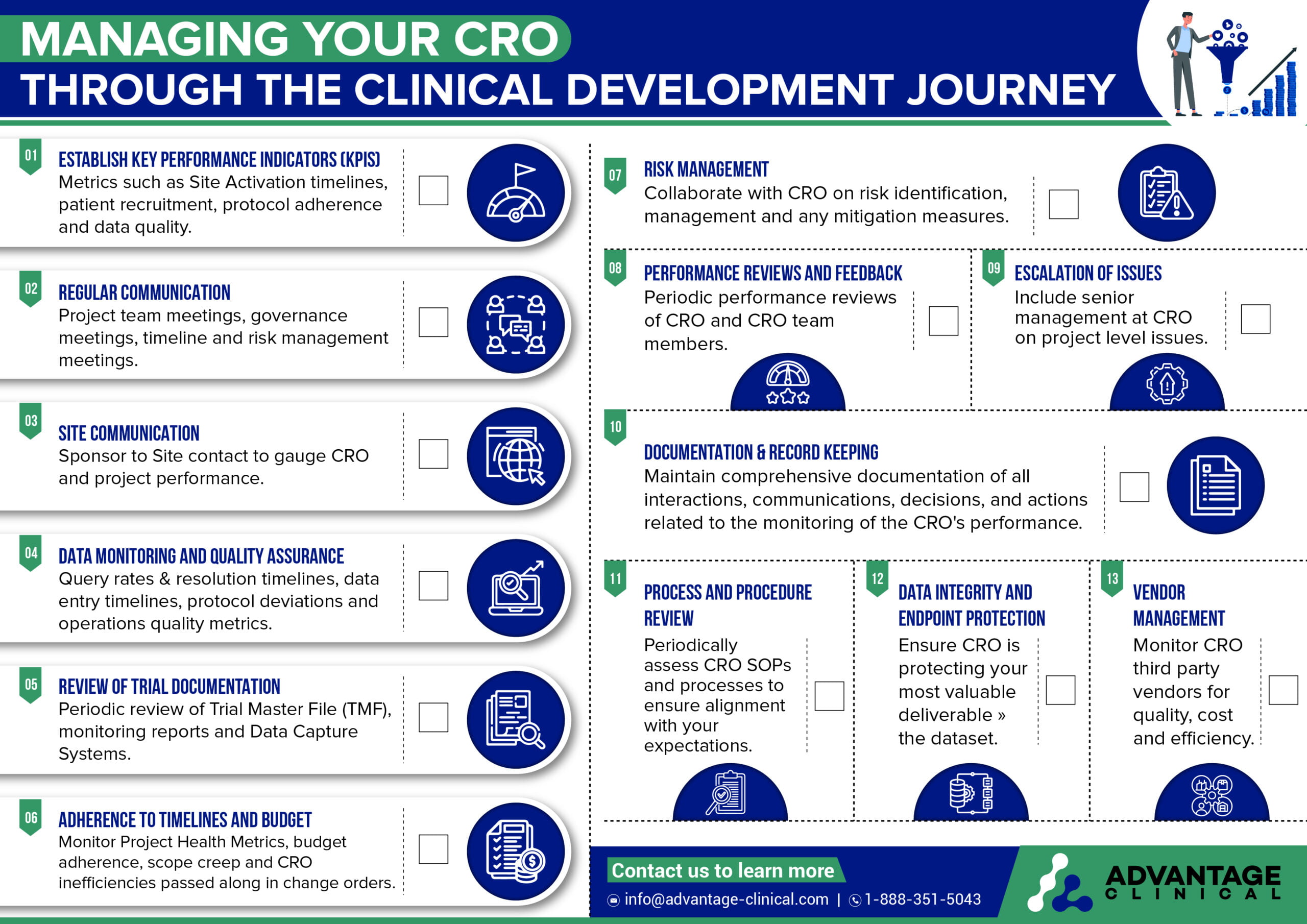
1 – Establish Key Performance Indicators (KPIs):
Before the trial begins, the sponsor and the CRO should agree upon specific KPIs to measure the CRO’s performance.
KPIs may include metrics related to site activation, patient recruitment, data quality, protocol adherence, timeline adherence, regulatory compliance, and budget management.
2 – Regular Communication:
Maintain regular communication with the CRO throughout the trial, including scheduled meetings, conference calls, and email correspondence.
Discuss project status updates, milestones achieved, challenges encountered, and any deviations from the agreed-upon timelines or deliverables.
In addition to project team meetings, it is encouraged to set project governance meetings with delivery directors, your account executive and other senior people at the CRO to ensure their leadership maintains oversight as well as has a read on your satisfaction of CRO performance on the study.
3 – Site Communication:
Clinical research sites are the boots on the ground for our clinical trials. They have frequent contact with the CRO including functional leads, project contributors and the Clinical Research Associates (CRAs). It is important to make sure sites are satisfied with their support from the CRO and speaking to them directly will help to uncover any challenges they are facing.
Site’s may also voice concerns about the protocol, study population, or other study operations issues which can be helpful in ensuring the study is working properly at all levels of the delivery chain.
4 – Data Monitoring and Quality Assurance:
While not all study data may be visible to the sponsor due to blinding restrictions, it is important to keep an eye on metrics which may be indicative of poor CRO / site performance. This may be query resolution timelines, data entry timelines, frequency of queries and protocol deviations etc.
Beyond site level data, sponsors can monitor metrics on the CRO such as trip report review / approval timelines, delays in investigator grant payments, site startup / activation timelines, monitoring plan compliance and more. These metrics may be indicators of resourcing or process issues at the sponsor and present an opportunity for both parties to work together to bring back into compliance with some of the KPIs set out at the start of the partnership.
5 – Review of Trial Documentation:
The sponsor should have access to the TMF, though sections may be blinded and inaccessible to the sponsor until the trial has concluded. Spot checks of the TMF may uncover missing / pending documents or document management practices that may not be in compliance with sponsor expectations.
Sponsors may also choose to review a selection of CRA Monitoring reports to see how things are performing at the site level from the eyes of the CRA. Note again some sections may be blinded from the sponsor and this should be confirmed by the CRO before trip reports are shared.
6 – Adherence to Timelines and Budget:
Monitor the CRO’s performance in meeting project timelines and milestones outlined in the contract and trial plans.
Evaluate budget management practices to ensure that the trial stays within budgetary constraints and that resources are allocated efficiently.
It is important to review all CRO billings to ensure that pass throughs, investigator grants and any unit / milestone billings are accurate.
CROs are quite good at managing scope creep, though the sponsor should make every effort to ensure they are staying in line with the agreed scope as well to prevent any clawback attempts later in the study.
Change orders are very common in this industry and it is important that the sponsor thoroughly reviews all change orders and looks for rationale for any increases in pricing, scope or units. This can be a lengthy process but it is important to get it right and not cave to pressures from the CROs to sign the change order within their expected timeframe ( generally leading up to the end of a fiscal quarter).
7 – Risk Management:
Risk management should be a collaborative process between the CRO and the sponsor and should include feedback from key stakeholders across both organizations.
Identify potential risks and issues that may impact the conduct of the trial and develop risk mitigation strategies in collaboration with the CRO.
Monitor the implementation of risk mitigation measures and assess their effectiveness in addressing identified risks.
Risk management meetings should occur regularly throughout the life of the study. We recommend at-least bimonthly as well as during any major study changes such as a protocol amendment.
8 – Performance Reviews and Feedback:
Conduct periodic performance reviews with the CRO to evaluate overall performance against established KPIs and objectives.
Provide constructive feedback to the CRO based on performance assessments and identify areas for improvement or corrective actions.
You are going to work closely with many of the team members at the sponsor. Be sure to provide feedback both constructive and positive to them as well as to the project leadership and governance groups.
9 – Escalation of Issues:
In the event of significant deviations, non-compliance issues, or concerns about the CRO’s performance, escalate the matter to senior management or relevant regulatory authorities as appropriate.
Implement corrective actions and follow-up measures to address identified issues and ensure the integrity and validity of the clinical trial data.
10 – Documentation and Record Keeping:
Maintain comprehensive documentation of all interactions, communications, decisions, and actions related to the monitoring of the CRO’s performance.
Keep accurate records of performance assessments, site monitoring visits, data reviews, and any deviations or corrective actions taken.
Summary
By implementing robust monitoring and oversight mechanisms, sponsor companies can effectively evaluate and manage the performance of Contract Research Organizations throughout the life of a clinical trial, thereby ensuring the successful and timely completion of the trial while maintaining high standards of quality and compliance.
Remember this may be the start of a long partnership between the sponsor and CRO and both parties want to keep they are receiving value from the collaboration. Setting up for long term success is key as running additional trials with the same CRO is much simpler as they have the background on the investigational therapeutic as well as your company.