Using a CRO- Risks, benefits and challenges. Can I do drug development in house?
Do I need a contract research organization or can we do this on our own?
Hiring a contract research organization (CRO) to execute on your clinical studies / program can be a daunting task. Finding the right partner will be invaluable and help to make the process as smooth as possible.
There are a number of benefits to working with CRO, and there are also a lot of risks along the way which need to be monitored and mitigated. Partnering with Advantage Clinical can help you to maximize your relationship with your CRO partner, to learn more visit our Services and Capabilities Page.
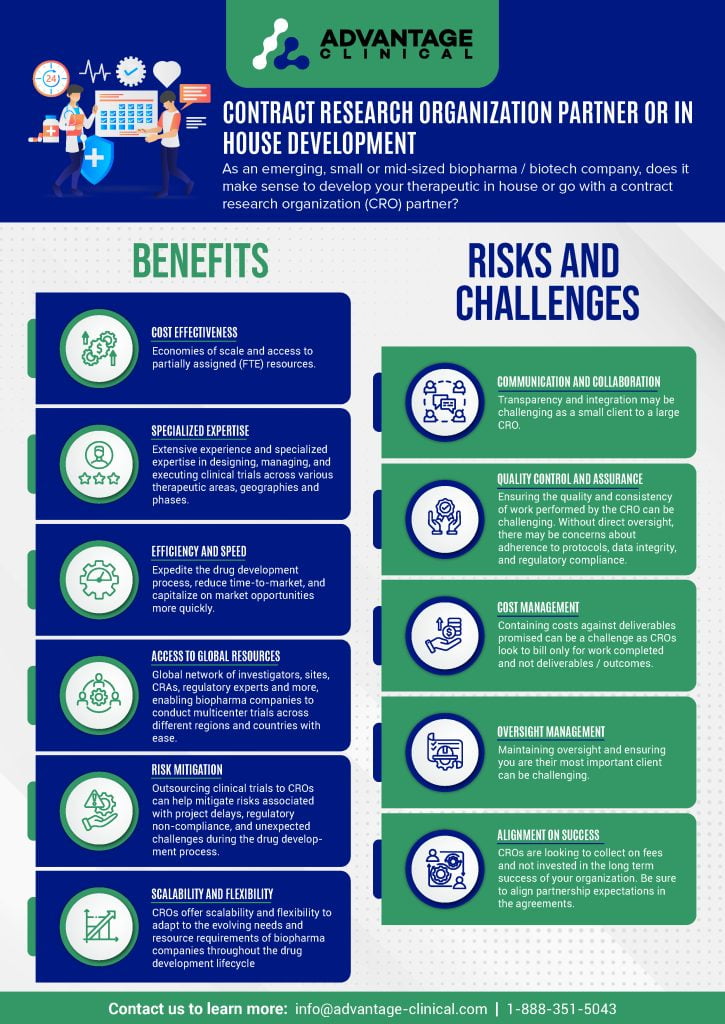
Benefits
Cost-Effectiveness:
Conducting clinical trials in-house can be prohibitively expensive for small biopharma companies due to the significant investment required in infrastructure, personnel, and resources.
CROs often offer flexible pricing models and economies of scale, allowing small companies to access specialized expertise and facilities at a fraction of the cost of building and maintaining their own infrastructure.
Specialized Expertise:
CROs have extensive experience and specialized expertise in designing, managing, and executing clinical trials across various therapeutic areas and phases.
Small biopharma companies may lack the in-house expertise and resources required to navigate the complex regulatory landscape, design robust study protocols, recruit patients, and ensure compliance with Good Clinical Practice (GCP) guidelines and local regulations.
Efficiency and Speed:
CROs are equipped with established processes, workflows, and infrastructure to streamline the conduct of clinical trials, leading to faster study startup times and accelerated recruitment of patients.
By outsourcing clinical trials to CROs, small biopharma companies can expedite the drug development process, reduce time-to-market, and capitalize on market opportunities more quickly.
Access to Global Resources:
CROs often have a global network of investigators, sites, and regulatory experts, enabling small biopharma companies to conduct multicenter trials across different regions and countries.
Leveraging the international capabilities of CROs can enhance the diversity of patient populations, improve data quality, and facilitate regulatory submissions in multiple jurisdictions.
Risk Mitigation:
Outsourcing clinical trials to CROs can help mitigate risks associated with project delays, regulatory non-compliance, and unexpected challenges during the drug development process.
CROs assume responsibility for project management, quality assurance, and regulatory compliance, allowing small biopharma companies to focus on core activities such as research, strategy, and business development.
Scalability and Flexibility:
CROs offer scalability and flexibility to adapt to the evolving needs and resource requirements of small biopharma companies throughout the drug development lifecycle.
Whether it’s scaling up to meet enrollment targets or adjusting study protocols in response to emerging data, CROs can provide tailored solutions to accommodate the dynamic nature of clinical research.
Focus on Core Competencies:
By outsourcing clinical trials to CROs, small biopharma companies can concentrate their internal resources and expertise on core competencies such as drug discovery, innovation, and commercialization strategies.
This allows companies to maximize their strengths, optimize resource allocation, and maintain a competitive edge in the rapidly evolving biopharmaceutical industry.
Risks and Challenges
Communication and Collaboration
Integrating two organizations with different cultures can be challenging. Ensuring up from alignment by choosing the right partner and developing robust communication protocols can be helpful in ensuring open and transparent communication throughout your project lifecycle.
Whether it’s your weekly project meetings, CRO responsiveness to email communications or their relationships with your research sites, building an atmosphere or collaboration will be key to long term success.
Quality Control and Assurance
While most CROs will have robust quality assurance and control system in place, these may not always align with your expectations on project quality. This extends through to project data. This is a major expenditure for your organization in terms of capital, resources and time. Ensuring a high quality dataset deliverable at study conclusion should be top priority. Working with the CRO closely throughout the project lifecycle to continually monitor data and project quality will help to ensure long term quality.
Cost Management
Most projects in our industry tend to run over time and over budget. CROs are a service provider and are looking to bill for services provided. When the project runs over they continue to get paid. Many projects can be plagued by change order after change order resulting in increasing timelines and costs for your organization.
Holding your CRO accountable for performance through the life of your project is key to containing costs. Billings and change order should be continually reviewed against the initial bid / proposal documents and contractual obligations to ensure inefficiencies at the CRO are not being passed onto you. This is a very common issues in our industry and is easily mitigated with the right level of oversight.
Oversight Management
Large CROs may be working on more than 1500 clinical projects at any given time. As a small or emerging biotech you will be competing for resources within the CRO against large pharma partnerships and many other organizations like yourselves. Ensure you stand out can be challenging.
Keeping continual oversight and open communication with the CRO and leadership there will help to keep you top of mind. Part of choosing the right CRO partner will help to mitigate many of these risks, but maintaining proper oversight long term is essential.
This can be challenging as there are a lot of moving pieces involved in project execution and delivery. For many organizations this will involve having an in house shadow team which works tightly with the CRO on project execution through oversight and collaboration. Working with Advantage Clinical can fill this role and maintain oversight on your behalf.
Alignment on Success
As we alluded to earlier, CROs are service providers and they get paid whether things go to plan or not. Ensuring alignment on success early in the relationship will help to hold them accountable for deliverables that matter to you.
This is often part of the contract negotiations and may include multiple penalty / bonus clauses. This could be something like “All sites activated by 01Jul2025, if early 3% bonus, if late 5% penalty”. This is not something CROs will often offer up, but with increasing competition for studies CROs are willing to compete with clauses such as these.
Conclusion
Leveraging the services of Contract Research Organizations offers numerous advantages for small biopharma companies, including cost-effectiveness, access to specialized expertise, efficiency, risk mitigation, and scalability. By partnering with CROs, small companies can accelerate the drug development process, enhance their competitiveness, and bring innovative therapies to market more effectively.
Choosing the right CRO partner and maintain oversight throughout the life of the study will give you the best chance of success in your clinical development journey.
Advantage Clinical specializes in CRO selection and management. With our deep knowledge of the CRO world we can be by your side along this journey to help make your project successful.



