The FDA’s latest draft guidance on artificial intelligence (AI) is setting the stage for how AI can be used to support regulatory decision-making in drug and biological product development. This is a significant step forward, offering clarity on how AI models should...
ICH E6(R3) Is Finalized! What’s Different from the Draft Guidance?
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has finalized the much-anticipated E6(R3) guideline on Good Clinical Practice (GCP). Released on January 6, 2025, the final version represents the culmination...
ICH Releases Draft Annex 2 for GCP E6(R3): Public Consultation Now Open
The International Council for Harmonisation (ICH) has taken another significant step toward modernizing Good Clinical Practice (GCP) guidelines with the release of the draft Annex 2 to GCP E6(R3). This latest addition is now available for public consultation and...
Understanding the New 2024 Declaration of Helsinki: Key Updates for Clinical Research
The World Medical Association (WMA) has recently unveiled a significant revision to the Declaration of Helsinki, a cornerstone document guiding the ethical principles of medical research involving human participants. This 2024 update brings critical enhancements to...
Strategic Planning for Clinical Research Enterprises
Why Your Clinical Research Organization Needs a Strategic Plan In the evolving world of clinical research, developing or updating a strategic plan is essential for keeping pace with industry advancements, regulatory changes, and operational challenges. Whether your...
Overview of ICH GCP E6 R3: Key Changes and What They Mean for Clinical Trials
Overview of ICH GCP E6 R3: Key Changes and What They Mean for Clinical Trials The release of ICH GCP E6R3 marks a significant update to the Good Clinical Practice (GCP) guidelines, reflecting the evolving nature of clinical trials. While the previous version, E6R2,...
WHO’s Global Guidance for Best Practices in Clinical Trials- Coming September 2024
WHO's Global Guidance for Best Practices in Clinical Trials In May 2022, the World Health Assembly (WHA) passed Resolution WHA75.8, aiming to improve the quality, coordination, and reliability of clinical trials across the globe. This resolution addressed the need for...
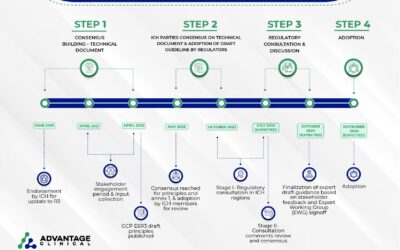
Good Clinical Practice (GCP) E6 R3 Development, Release Date and Adoption
The International Council for Harmonization (ICH) proposed the updated to GCP from E6R2 to E6R3 back in June 2019. Fast forward to today as we are approaching the implementation of E6R3 sometime in Q4 2024. The ICH works to update their guidelines in a very structured...
Managing the CRO Through the Clinical Development Journey
Managing the CRO Through the Clinical Development Journey Monitoring a Contract Research Organization's (CRO) performance throughout the life of a clinical trial is crucial to ensure that the trial is conducted efficiently, ethically, and in compliance with regulatory...