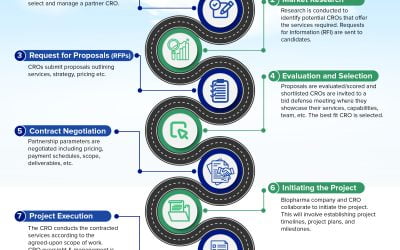
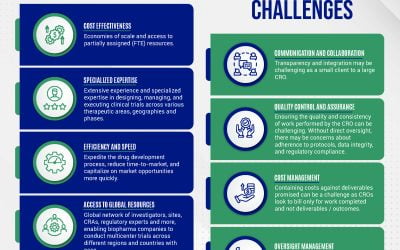
Understanding the CRO Selection & Management Process Small emerging biopharma companies typically choose a contract research organization (CRO) based on several key factors, including expertise, capabilities, reputation, cost, and flexibility. Below is an overview...
Do I need a contract research organization or can we do this on our own?
Do I need a contract research organization or can we do this on our own? Hiring a contract research organization (CRO) to execute on your clinical studies / program can be a daunting task. Finding the right partner will be invaluable and help to make the process as...
How do I get started? A Crash course on bringing new therapies to market
How do I get started? A Crash course on bringing new therapies to market Starting a pharmaceutical company and bringing a drug to market is a complex process that requires significant expertise, resources, and adherence to regulatory requirements. Here's a general...
A Critical Look at Inclusion & Exclusion Criteria
Based upon US FDA Workshop Report April 16 2018 In response to the FDARA( Food and Drug Reauthorization Act) of 2017, the US FDA recently convened a workshop to evaluate inclusion & exclusion criteria in clinical trials. This workshop was attended by...
Developing a Training Program at your Institution- The 7A Model
So you need to implement a training program at your organization…where do you even begin? Training/learning programs can exist in a number different forms. Sometimes they are a collection of courses, documents, and training classes that have been developed over a...
Expanding Technology in Clinical Trials Fosters Process Improvement
The pharmaceutical sector has been slow to adopt technologies designed to boost efficiencies in the very complex business of developing new therapies (Chart 1). Fortunately, this is changing as sponsors and contract research organizations embrace technologies that...
FDA Common Rule- A Much Needed Update
In a sweeping gesture to better protect human research subjects, sixteen US federal agencies issued a revised Common Rule on January 19, 2017, the first update since 1991. The final rule, officially known as Federal Policy for the Protection of Human Subjects, aims...
FDA Releases GCP E6 R2 Guidance
Released by the International Council on Harmonisation (ICH) in November 2016, Good Clinical Practice’s second revision, R2 has been making waves in the clinical research industry. In March 2018, the US FDA released E6(R2) Good Clinical Practice: Integrated Addendum...
Clinical Research Conferences 2018
Which Clinical Research Conferences to Attend in 2018? Happy new year and welcome to 2018! As the clinical research industry continues to grow so does the plethora of educational opportunities and conferences that cater to our industry. Clinical Research is a very...
Quality Management in Good Clinical Practice (GCP) E6 R2 Update
Good Clinical Practice GCP E6 R2 Update The largest addition to Good Clinical Practice in Release 2 is the addition of Quality Management ,section 5.0, which falls under Sponsor guidance. Although this section of the guidance is directed to the sponsor audience,...
Investigator Oversight: Changes in Good Clinical Practice November 2016 Update
In the first update to Good Clinical Practice E6 since 1996, Release 2 introduced 26 major additions to the gold standard for the conduct of clinical research. Release 2 was finalized in November 2016, sites and sponsors must now must bring themselves into compliance...
GCP E6 R2 Addendum; Impacts for Investigators and Clinical Research Sites
Adopted in November 2016, the second revision to ICH Good Clinical Practices E6 R2, is the biggest revision to GCP in 20 years. The addendum was crafted partly in response to the concerns raised from hundreds of international GCP regulatory inspections. Some of the...
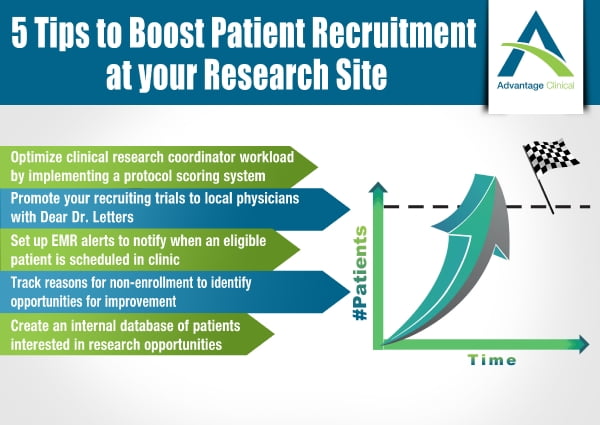
5 Tips to Boost Patient Recruitment at your Research Site
As a clinical research site you are undoubtedly constantly being approached by your sponsors inquiring about enrollment at your centre. Being a site which can efficiently and consistently meet recruitment quotas is a stellar quality and will have sponsors knocking on...
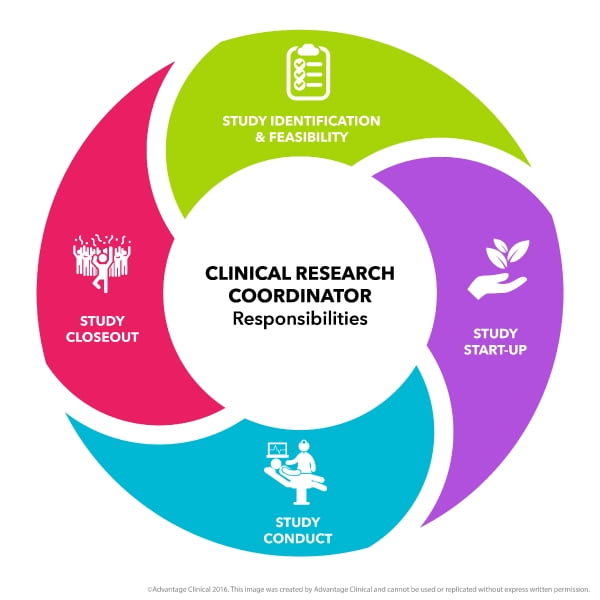
Protocol Scoring and Determining Study Load per Clinical Research Coordinator
Due to the increasing complexity of clinical research protocols and the need to justify staffing, it is critical for clinical research sites to have methods to evaluate, quantify,
6 Tips to Boost Patient Recruitment in your Clinical Trial
Sluggish patient recruitment is costing the clinical research industry hundreds of millions of dollars in missed opportunity costs.
Mastering the Informed Consent Discussion
Informed consent is the process through which a potential subject is informed about a clinical research study and volunteers to
Leveraging Clinical Trial Pre-Screening and Screening Data
Sponsors have long debated whether or not to collect pre-screening and screening data for sites participating in their trials. Understandably this is a burden on the site, having to record screening efforts in real time and upload them to the sponsor on a weekly, bi-weekly or monthly basis. However, the utility of this data in shaping and pivoting your recruitment program in invaluable. Beyond the use of this dataset in the current trial, sponsors can leverage this data to better understand patient populations in this indication as well as better assess sites for future trials.
Utilizing a Patient Recruitment Specialist
The Patient Recruitment Specialist (PRS) is a fairly new and emerging role in the clinical research industry. Their role varies between trials and sponsors though their primary duty